This is post number four of our six part post on Pharmacology. Our posts will focus on the following topics:
Part 1. Introduction
Part 2. Pharmacokinetics
Part 3. Pharmacodynamics
Part 4. Bioavailabilty
Part 5. “Free versus Bound Drug”
Part 6. Elucidating Pharmacodynamic Effect from an Analytical Chemistry Result
We talked in Part 2 Pharmacokinetics about the concept of absorption. Simply put, absorption is the movement of a drug into the bloodstream from the site of administration (oral, IV, subcutaneous, nasal, etc.). It answers the question of how it goes from the pre-consumption form of the drug into our blood for later potential affect on the human or possible detection in the blood if sampled. When dealing with DUID cases, it is essential to know a particular important part of absorption called bioavailability.
Broadly defined bioavailability is used to describe the fraction of an administered dose of unchanged drug that reaches the systemic circulation. If the drug was administered by mouth, the fraction that got into the blood could be determined by comparing to the same dose given directly into the blood, i.e., intravenously (IV).
By definition, drugs that are administered by way of Intravenous (IV) delivery have a bioavailability of 100%. What becomes trickier are those drugs that are not administered by IV, but by another route of administration. For chemicals taken by routes other than the IV route, the extent of absorption and the bioavailability must be understood in order to determine whether a certain exposure dose will induce impairing or even toxic effects or no effect whatsoever. The concept of bioavailability may, in part, also explain why the same dose may cause toxicity by one route (IV) but not the other (smoking).

Examples of drugs and their different bioavailability:
- Oxycodone has a bioavailability of about 60–87% when administered orally; rectal administration is reported to be about the same; and when administered by intranasal means varies between individuals with a mean of 46%.
- Propranolol has a bioavailability of about 26% because 75-85 % is metabolized by the liver before it can reach the circulation when taken orally.
- Morphine has a bioavailability of about 30% because 70% is metabolized via 1st pass effect (metabolism by the liver) if taken orally. Morphine is therefore usually given intramuscular (IM) injection to bypass this mechanism.
- Codeine-has a bioavailability of about 90% when administered orally.
- Cocaine-has a bioavailability of about 33% when taken orally; when administered by intranasal means is about 60–80%; and when given by nasal spray is about 25–43%.
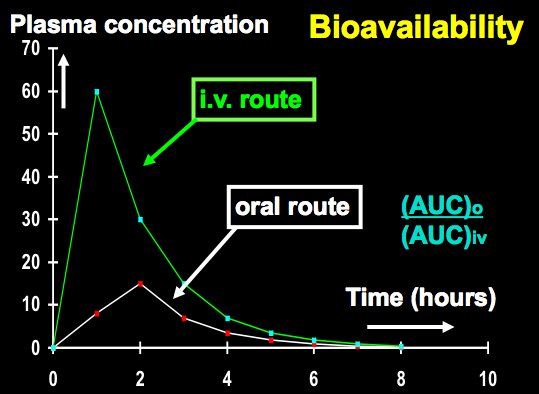
- Bioavailability IV versus oral
There are many factors that affect the bioavailability of the orally administered drug. This includes, but are not limited to:
- Rate of dissolution (breakdown) of the drug formulation – liquids>solids
- Surface Area of the drug formulation – micro-particle size has greatest surface area
- pH in the gastrointestinal tract – if a drug is ionized it will not be absorbed well from the GI tract
- Fat (lipid) Solubility – the more lipid soluble the greater is absorption
- Concentration administered – smaller doses tend to be more bioavailable than large dose
- Food – drugs compete with food in the GI tract for absorption; the more food present the slower the absorption
David M. Benjamin, Ph.D. (Clinical Pharmacologist) writes:
Some Additional Insight into Bioavailability and Pharmacology
Bioavailability is a measure of both the rate of absorption and the extent of absorption. The rate of absorption is determined by calculation of the Tmax, or the time required to reach the peak drug blood concentration (Cmax), and the extent of absorption is determined by calculation of the area under the curve (AUC). Regardless of the shape of the AUC curve, (ie, fast or slow Tmax, low or high Cmax) if the areas inder the curves are the same, the same amount of drug was absorbed. This can be determined easily by cutting out the curves and weighing them on a sensitive scale, or by integral calculus, for you math nerds.Many factors impact on bioavailability. An oral dose must first disintegrate and then dissolve in gastric or enteric juices. Most drugs are far better absorbed from the first 12 inches of the small intestines called the duodenum, from the Latin word for 12 (duodecum). Therefore, factors such as food and low gastric pH (indicating high acidity) slow down gastric transit time from the stomach into the intestines and the rate of absorption is slowed down. This phenomenon is well established with ethanol, which also has the extent of absorption decreased by food in the stomach, since the ethanol gets trapped and binds to food and just continues past the duodenum to lower small intestine sites (jejunum and ileum) where absorption is less than in the duodenum.
Oral morphine is poorly absorbed by oral administration, but it is probably due more to its water (aqueous) solubility than a first-pass effect. The morphine molecule has both an alcoholic hydroxyl group (-OH) on the 6 position and a phenolic hydroxyl (-OH) group on the 3 position of the aromatic or benzenoid ring. The OH groups confer water solubility and the the drug is not able to permeate the predominantly lipid-soluble (fat-soluble) membranes of the GI tract. Addition of a methyl group (-CH3) to the 3 position changes morphine to codeine, masks the OH group and confers greater fat-solubility to the molecule allowing it to be more preferentially absorbed. However, it does have to be de-methylated back to morphine (in the liver) to expert its analgesic properties. This is done by the CYP 2D6 microsomal enzyme of which there are 5-6 pharmogenetically active forms ranging from ultra-slow to ultra-fast. People who can’t metabolize codeine efficiently, also can’t metabolize dexromethorphan (Robitussin cough suppressant) well, and can become impaired on it on suffer dissociative reactions. There have been many arrests for DUI-Dextromethorphan.
Once absorption is complete, morphine has to be distributed to the brain (Central Nervous System; CNS). Due to its water solubility, it has been estimated that only one molecule of morphine out of 1,000 molecules circulating in the blood actually gets into the brain to relieve pain and exert its array of pharmacologic activities. This is not bioavailability, but distribution from blood to the site of the organ where the receptors are located.



David M. Benjamin, Ph.D. says:
Some Additional Insight into Bioavailability and Pharmacology
Bioavailability is a measure of both the rate of absorption and the extent of absorption. The rate of absorption is determined by calculation of the Tmax, or the time required to reach the peak drug blood concentration (Cmax), and the extent of absorption is determined by calculation of the area under the curve (AUC). Regardless of the shape of the AUC curve, (ie, fast or slow Tmax, low or high Cmax) if the areas inder the curves are the same, the same amount of drug was absorbed. This can be determined easily by cutting out the curves and weighing them on a sensitive scale, or by integral calculus, for you math nerds.
Many factors impact on bioavailability. An oral dose must first disintegrate and then dissolve in gastric or enteric juices. Most drugs are far better absorbed from the first 12 inches of the small intestines called the duodenum, from the Latin word for 12 (duodecum). Therefore, factors such as food and low gastric pH (indicating high acidity) slow down gastric transit time from the stomach into the intestines and the rate of absorption is slowed down. This phenomenon is well established with ethanol, which also has the extent of absorption decreased by food in the stomach, since the ethanol gets trapped and binds to food and just continues past the duodenum to lower small intestine sites (jejunum and ileum) where absorption is less than in the duodenum.
Oral morphine is poorly absorbed by oral administration, but it is probably due more to its water (aqueous) solubility than a first-pass effect. The morphine molecule has both an alcoholic hydroxyl group (-OH) on the 6 position and a phenolic hydroxyl (-OH) group on the 3 position of the aromatic or benzenoid ring. The OH groups confer water solubility and the the drug is not able to permeate the predominantly lipid-soluble (fat-soluble) membranes of the GI tract. Addition of a methyl group (-CH3) to the 3 position changes morphine to codeine, masks the OH group and confers greater fat-solubility to the molecule allowing it to be more preferentially absorbed. However, it does have to be de-methylated back to morphine (in the liver) to expert its analgesic proerties. This is done by the CYP 2D6 microsomal enzyme of which there are 5-6 pharmogenetically active forms ranging from ultra-slow to ultra-fast. People who can’t metabolize codeine efficiently, also can’t metabolize dexromethorphan (Robitussin cough suppressant) well, and can become impaired on it on suffer dissociative reactions. There have been many arrests for DUI-Dextromethorphan.
Once absorption is complete, morphine has to be distributed to the brain (Central Nervous System; CNS). Due to its water solubility, it has been estimated that only one molecule of morphine out of 1,000 molecules circulating in the blood actually gets into the brain to relieve pain and exert its array of pharmacologic activities. This is not bioavailability, but distribution from blood to the site of the organ where the receptors are located.
David M. Benjamin, Ph.D.
Clinnical Pharmacologist
Justin J. McShane says:
Thanks Doc. We will add this to the main post. Great information. Please feel free to add whenever there is good and detailed information to add. Thanks!