Why do I see two peaks on my EtOH analysis using HS-GC-FID?
This is a frequent question asked by criminal defense lawyers. Typically in the analysis of an accused’s motorist’s blood for EtOH composition (his or her Blood Alcohol Content) by way of Headspace Gas Chromatography with Flame Ionization Detector (HS-GC-FID) we will see two peaks if EtOH is alleged to be present. It can look like the below:
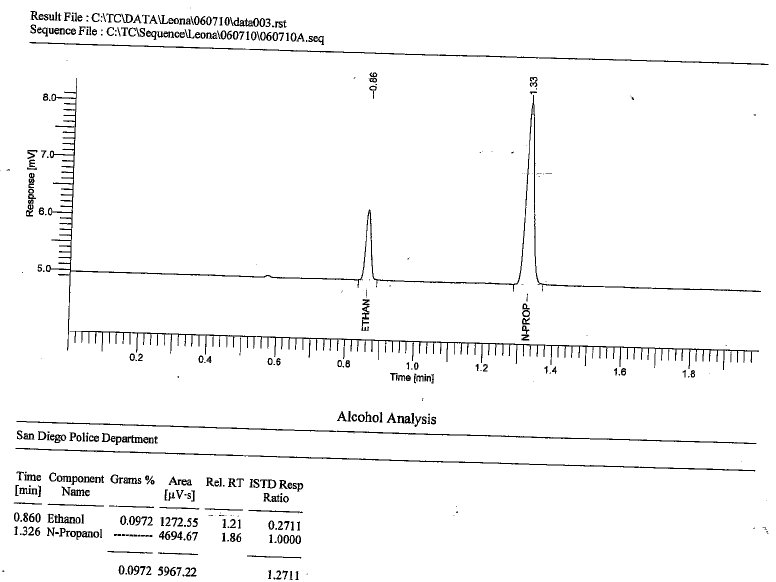
What is that second peak?
The two peaks exist because in the method, the analyst uses what is referred to as an Internal Standard (ISTD). An ISTD is a known and static amount of an analyte that is purposefully spiked (added) to the solution (blood). In other words, it goes into every single headspace vial in the run (unless there is a true blank-aka aqueous blank). It is added during sample preparation time using volumetric delivery devises such as pipettes.
The challenge to using the ISTD method is to find an analyte that is similar to but not likely organically present within the blood. This is why in EtOH determination n-propanol is generally used as the ISTD. N-propanol’s chemical formula is CH3CH2CH2OH, and is therefore structurally similar to EtOH (CH3CH2OH) in that they are both alcohols, just one CH2 different. As a result, as Gas Chromatography is primarily a separation technique based upon boiling point and polarity in conjunction with the analyte’s affinity to the particular stationary phase of the column, the two compounds (EtOH and n-prop) will be easily separated with significant resolution (separation) as seen above. With most GC columns used for EtOH determination, n-prop will elute (come put) second in time over that of EtOH. What makes n-prop a “good” selection to use as an ISTD is that it is primarily used as a solvent in the pharmaceutical industry, and for resins and cellulose esters, and therefore is not naturally present in a living human beings blood. (It is part of the body’s natural cellular decomposition that occurs after death. So, n-prop would be a “poor” choice for EtOH analysis in post-mortem blood. Instead, MEK is preferred).
Why use the ISTD method?
The purpose of adding this known and static amount is to help insure accuracy (meaning attempting to insure against deviations of bias). By adding this known and static amount of the ISTD, we can try to guard against pipetting errors in the sample preparation that without the ISTD would cause error. In the GC run, there should be an n prop blank that looks like the below:
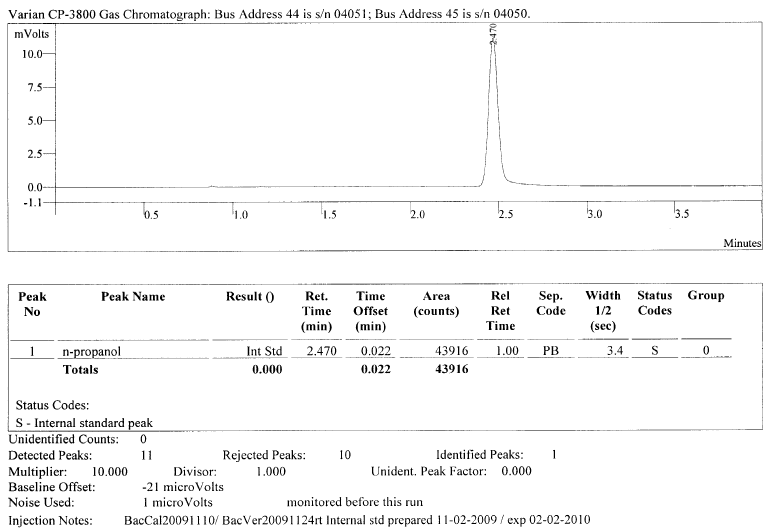
In a well-designed method this n-prop blank serves two purposes.
- First, it exists as a “retention time lock” for qualitative measure quality control. In our example above, the machine is now taught that anything that elutes (comes out) during the 2.470 minute period must be n-prop and n-prop alone. (Of course, this may not be true as there are 56 million organic and inorganic substances and 62 million sequences registered in CAS, but nevertheless this is what the machine is taught).
- Second, the area count of that peak serves as the amount that is the “area count target amount” or the amount that we want and expect that if there is good pipetting and good injecting by the analyst. It becomes our standard amount against which we compare all other chromatograms. In our example above, 43916 is the area count if everything is done “right” meaning that a static and known amount is added to each and every headspace vial. So, we can literally flip through each chromatogram of the run and see if any differ from this 43916 amount. Again, the goal is to have no deviation from this area count target amount for n-prop from chromatogram-to-chromatogram which would mean that there was no deviation in adding n-prop from vial-to-vial as we are trying to be dead-on precise with our delivery of n-prop. However, deviation is “normal.” Even the most conscientious person will cause deviation. Not even the most perfect machine will be able to deliver EXACTLY the same amount of n-prop into each headspace vial. If there is significant deviation (each method should have this well-defined in its instructions) in this ISTD area count number (in our example 43916), this is where a “correction” occurs. If the remaining vials deviate from that area count target, then a ratio correction occurs where everything is scaled up or down to get to “normal” (our area count target amount). So, if there is “too much” in the area count when we measure the next vial when it looks to the n-prop amount, it reduces the n-prop until it comes to “normal.” This resulting reduction is then calculated and becomes a ratio. But, it doesn’t just stop at correcting the n-prop amount, it similarly reduces any other analyte detected by that same correction ratio. So, if there is 20% “too much” n-prop detected, then the n-prop and everything else detected (including the EtOH) is reduced by 20%. If there is “too little” of the n-prop, the n-prop is scaled up to “normal” and every other analyte is also scaled up in that same ratio. So, if there is 20% “too little” n-prop detected, then the n-prop and everything else detected (including the EtOH) is increased by 20%. In short, if the area count for the ISTD remains constantly the same (meaning it is not outside of the acceptance criteria), then there is no problem that there needs to be ratio corrected to account for. However, if there is significant deviation, then ratio correction is applied across the sample and everything detected.
Therefore, the key measure in terms of bias control is the measurement of the ISTD which in the case of EtOH determination n-prop.
Why isn’t the ISTD method used correctly in forensic science?
As we can see the ability of the detector to correctly quantify the n-prop is paramount, but guess what forensic labs never do? You got it. They never run a calibration curve for n-prop. As we discussed before (Why do instruments need to be calibrated? and When is a straight line a curve: Calibration curve and Personal bias is bad, but analytical bias spells disaster in DUI BAC and Drugs of Abuse testing and How do they make the squiggly lines turn into a magic number: Area under the peak and When is a straight line a curve: Calibration curve), without establishing a demonstrated linear dynamic range using CRMs on a well-constructed 5×5 calibration curve, then any measure that is other than the “area count target amount” and then applying ratio correction as described above is just plane wrong. A single point calibration curve (which is what is used in the above scenario with the 43916) is scientifically wrong. It presumes that any other measure is both accurate and precise which is not necessarily the case. Until they start doing this right and demonstrating scientifically the linearity of their response in the measure of the ISTD on the FID, then forensic laboratories are not truly performing science.


