This is post number five of our six part post on Pharmacology. Our posts will focus on the following topics:
Part 1. Introduction
Part 2. Pharmacokinetics
Part 3. Pharmacodynamics
Part 4. Bioavailabilty
Part 5. “Free versus Bound Drug”
Part 6. Elucidating Pharmacodynamic Effect from an Analytical Chemistry Result
When blood sample is analyzed for purposes of trying to determine possible impairment in a forensic context or therapeutic versus toxic monitoring in clinical pharmacology, the total amount of the drug in the bloodstream is that which is recorded (if the analysis is correct). However, the large question becomes,
Is this a truly relevant measure?
No.
Shocking as that may seem at first, the truth is that if you are trying to determine possible impairment or therapeutic versus toxic monitoring, then we need to understand the concept of “free” versus “bound” drug form. Otherwise, if we do not, we may misinterpret the pharmacodynamic effect based upon an analytical measurement. We need to examine drug distribution in conjunction with this concept of “free” versus “bound” drug form to form a complete intelligent picture that involves the concepts of Pharmacokinetics, Pharmacodynamics, and Bioavailabilty.
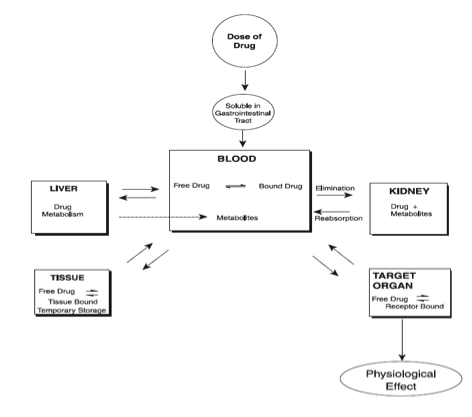
- Drug Disposition
For any ingested drug, it initially enters the stomach, usually dissolves, and is then absorbed into the bloodstream. Next, the absorbed drug circulates in the vascular compartment. An equilibrium mixture of free and protein-bound forms is achieved as it courses through the body.
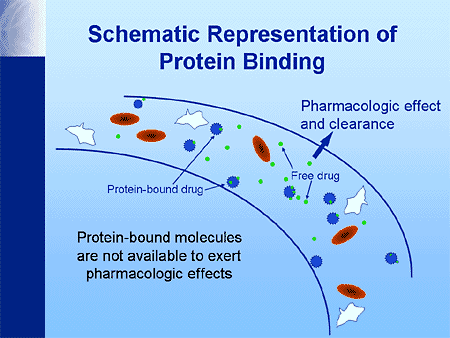
What is of particular note is that drugs can bind to plasma proteins (usually albumin). While the binding of drugs is reversible, the binding affinity of drugs is variable. Anionic drugs, which are weak acids, and hydrophobic drugs, and highly polar drugs have the strongest affinity for binding to these proteins. If there are two or more drugs in the bloodstream both with a high affinity to bind, they will actually compete to bind against each other. Only unbound forms of the drug (otherwise known as the “free” form of the drug) can act on target sites in the tissues, and may cause pharmacology-relevant effect. On the other hand, bound drugs are always pharmacologically inactive because they are not free to enter the tissue. The brain is the tissue of particular concern as that is the epicenter of impairment. The drug produces symptoms, such as impairment, depending strictly on the tissue content. In a living person, we cannot sample the brain to get an accurate measure of the free form that is in that tissue. In other words, we cannot measure blood content of drug in the tissue (brain). Instead we must measure what is in the bloodstream which is both the free and the bound form. The blood sample testing by way of analytical chemistry cannot differentiate (discriminate) between the free and the bound form. It is just a gross (aggregate) measure of both the free and the bound form.
So, as the analytical chemistry analysis of the blood measures both the “free” and the “bound” form, and only the free form has the possibility of being pharmacologically active, then we need to be particularly aware of the ratio that a drug has between free and bound to begin to possibly understand the possibility of impairment.
Of particular concern are chronic users of drugs. For example, very nonpolar drugs (termed fat or lipid soluable) are stored for varying intervals in fat tissue. [Note, metabolites are always more polar than parent drugs. As such, there is no such thing as a nonpolar metabolite.] They can then later re-enter the blood from the fat tissue. If the concentration of drug and metabolite within the blood is high, as would be the case for a chronic drug user, then the equilibrium favors continued adipose (fat) tissue storage. This makes later release into the bloodstream despite actual abstinence a possibility. Again, as the analytical chemistry result, as typically processed (There are some extra pre-analytical methods available that can be used to separate free from bound drug forms. However, these processes are infrequently performed.), contains a measure of both free and bound (and there is no distinction between the two) this release from fat tissue could easily be misinterpreted and provide for a false positive despite abstinence.
Marilyn A. Huestis, Ph.D., has written extensively about this type of issue with marijuana. She and her colleagues have noted that in their research “[e]xceptionally long detection times have been reported for cannabinoid metabolites in the urine of frequent drug users during abstinence. During the terminal elimination phase, an individual may produce consecutive specimens that test positive, negative, and positive again over time.” (Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2587336/)
Other research that has noted this includes
- Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, Huestis MA. Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009 Oct;33(8):469-77.
- Dackis CA, Pottash AIC, Annitto W, Gold MS. Persistence of urinary marijuana levels after supervised abstinence. American Journal of Psychiatry. 1982;139:1196–1198.
- Kielland KB. Urinary excretion of cannabis metabolites. Tidsskr Nor Laegeforen. 1992 May 10;112(12):1585-6.
- Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, Cadet JL, Huestis MA. Do Delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009 Dec;104(12):2041-8. Epub 2009 Oct 5.
- Kelly P, Jones RT. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. Journal of Analytical Toxicology. 1992;16:228–235.
- Swatek R. Marijuana use:persistence and urinary elimination. Journal of Substance Abuse Treatment. 1984;1:265–270.
- Johansson E, Halldin MM. Urinary excretion half-life of delta1-tetrahydrocannabinol-7-oic acid in heavy marijuana users after smoking. Journal of Analytical Toxicology. 1989;13:218–223.
- Ellis GM, Mann MA, Judson BA, Schramm NT, Tashchian A. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clinical Pharmacology & Therapeutics. 1985;38:572–578.
- Hawks RL. Developments in cannabinoid analyses of body fluids: implications for forensic applications. In: Agurell S, Dewey W, Willette R, editors. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. Academic Press; 1983. pp. 1–12.
Psychotropic drugs such as tranquilizers, antidepressants, antipsychotics, mood-altering agents, etc., also create their own unique problems. These drugs effect users by binding at sites within the central nervous system (CNS). Because the CNS is quite remote from the blood, such psychotropic agents are frequently not suitable subjects for drug monitoring.
So, in summary, an accused motorist who insists that he or she had not taken an impairing drug yet who has a positive blood analysis for an impairing substance (and in particular marijuana or other very nonpolar drugs and nonpolar drug metabolites that may be stored for varying intervals in fat tissue) may be telling the truth due to the above.


