A frequently asked question on the listservs that I belong to basically asks the following question:
In GC-FID use to quantify Blood Alcohol Content where EtOH is the target analyte, how does the machine arrive at the reported number?
The machine is called a Gas Chromatograph with a Flame Ionization Detector (GC-FID). Typically the sample is introduced by way of headspace. The most frequently used technique is technically called Static Headspace Isothermal Wall Coated Open Tubular Gas Chromatography with Flame Ionization Detector.
First a crash course on the Flame Ionization Detector (FID).
The FID is the part of the apparatus that quantitates the result of the chromatographic effluent. Remember that the number one rule of GC-FID is that you must demonstrate with data (actually prove) proper resolution first. The measure of qualitative selectivity (separation) must be proven before we can validly quantitate.
The FID actually is a destructive, mass counting device. It literally burns all carbon-hydogen bonds (C-H bonds) from what comes off the column. The current is sensed by an electrometer, converted to a digital form and sent to an output device that gives us the peak. It counts the increase in the number of ions between a cathode and a diode. A polarizing voltage attracts these ions to a collector located near the flame. This current is measured with a high-impedance picoammeter. An electrometer is an electrical instrument for measuring electric charge or electrical potential difference. The response is proportional to the number of C-H bonds. So, therefore, identical amounts of methanol, ethanol, butanol and hexanol would not give equal area count responses. The FID itself emits an analog (constant) signal; yet, it is reported through a computer system that is digital in nature.
There are two main methods of determining quantitation when it comes to GC-FID: (1) Peak height, and (2)Peak area.
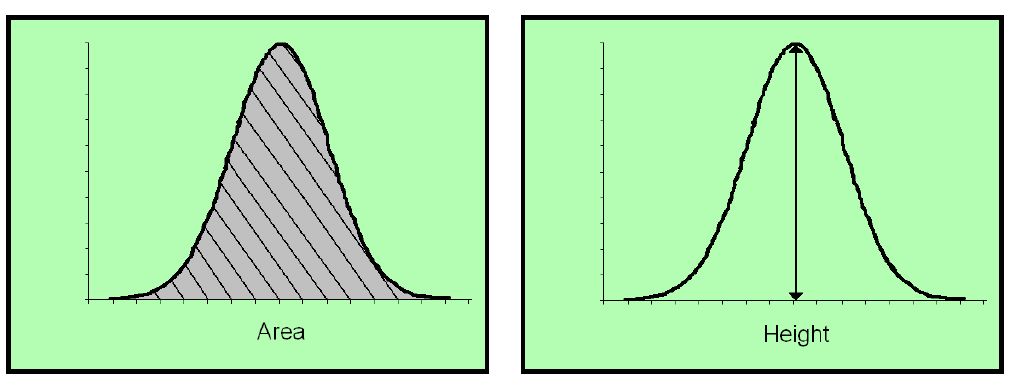
Peak height is an antiquated method of quantification that is not used a lot. It harkens back to a time when there were no computer programs. LabCorp and certain labs in California still use the peak height method. Both are based upon the standard dose response calibration curve.
In order to determine either peak height or peak area, the crucial boundary is the baseline.
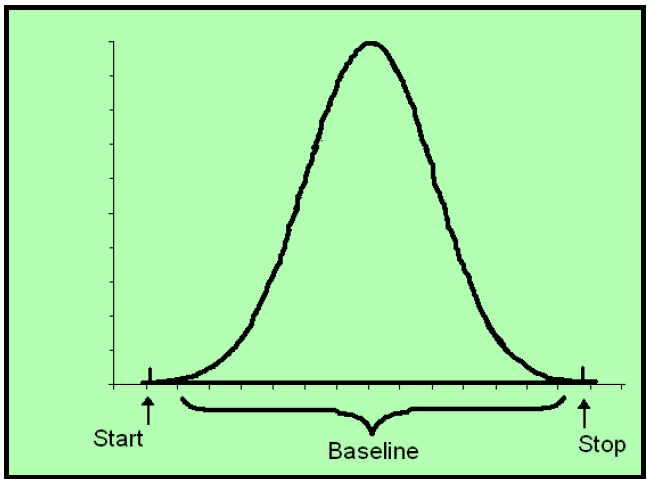
It is essential to remember that all detectors have analytical noise. There is no way of recording zero. No machine can measure true zero. There is always going to be an offset. As an aside, this is why on a calibration curve zero (the origin) cannot be used as a legitimate data point in determining the slope which gives us the quantitation.
While there is a peak, there is, of course, signal. As a result during the act of detection there is no baseline per se. Instead, the computer uses an algorithm based upon prior input before the peak and “guesses” what the baseline would be if there was no signal. As this is the computer’s guess, is it is subject to interpretation and error.
When we use the peak area method of quantitation, then the question becomes not only where is the baseline, but where does the computer begin to calculate the beginning and ending of a peak. This is so very important.
As you can see in the above there are vertical hash marks that are labeled “start” and “stop,” these are called “tick marks.” This is the other perimeter that determines peak area.
So in sum the determination of the peak area is a function of 3 bounds: (1) the baseline and (2) starting tick mark, and (3) ending tick mark. Think of them as boundary marks.
There are two types of integration: (1) auto-integration and (2) manual integration.
If the button pusher uses auto-integration, then the determination of the baseline will be calculated the same throughout the run. This is what should be done (provided that the use of the auto-integration events is static and equally applies to the calibrators and the unknowns that are tested and is part of a truly validated method).
On the other hand, one can use manual integration events to manipulate the data. This is a function of the end user. This is typically a one-time event in that only one chromatogram is manipulated. It may be something that can be discovered by looking at the chromatogram or it may be utterly undiscoverable unless you have the raw data. (Here is a video that shows this concept: The case for raw data: “Integration” in Gas Chromatography: How to make an innocent person guilty in a DUI case by manipulating the software Actually at Axion, we discovered another 3 ways of manipulating the raw data so it would be undetectable.)
The key function of all of this is that everything remain the same in order for the result to be valid. The integration events must be static in order for the quantification to be valid.
When looking at the EtOH peak:
The unknown that is your client’s sample must use the same integration perimeters as the calibrators. The boundary marks used to determine the peak area for the calibrators in their individual chromatograms must match that of the unknown. If the baseline remains the same, but the tick marks are wider for the calculation of the unknown than the calibrators, then this will over-report the Blood Alcohol Content (BAC). The converse to this is true as well. If the tick marks for the calibrator and the unknown remain the same, but the baseline for the unknown is lower than the calibrator, it will over-report the BAC. The converse is true as well.
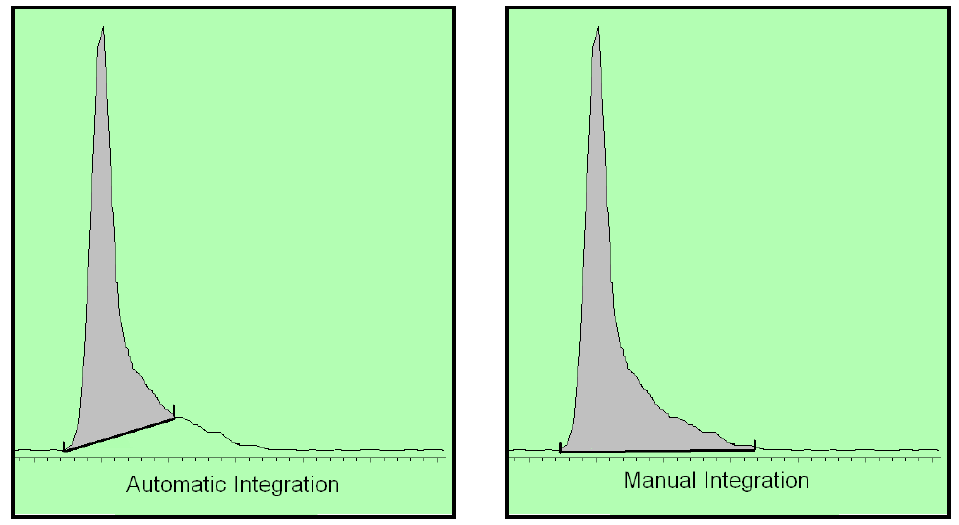
Above is an example of how the auto-integration leads to less area and how the manual integration leads to more area. More area equates a higher BAC if this is performed on the EtOH peak.
What further complicates this is the use of the Internal Standard (ISTD). The integration events for the ISTD must also remain static. A peak is a peak is a peak. The same boundary marks apply. However, with respect to the ISTD, the ISTD amount is inversely related to the EtOH. If there is too much ISTD calculated, then the BAC will be ratio reduced. The converse is true as well.
How can you tell if any of this is done? Well, maybe you can and maybe you can’t as discussed above and in the video. If you trust the laboratory, and if they report the baseline and the tick marks on all of the chromatograms, you can simply use a lightbox or even hold it up to a light with the calibrators and the unknowns on top of one another and see if the boundary marks are all treated the same.
Bottom line take away: Consistent and appropriate integrations are scientifically defensible. Inconsistent or inappropriate integrations are difficult to defend. All QC, Verifiers and Unknowns must be treated in a consistent (same) manner and per a validated method. Otherwise, you have a non-validated result.
The take away question: What does your laboratory do when it reports out it’s BAC?



Anjum Shahzad says:
Respected Sir,
how can we change the intergration by manually. if you have some data related to Intergation events for GC. Please send me although i can study and discuss with you.
Thanks.
Justin J. McShane says:
Here is an entire youtube video on this concept. https://www.youtube.com/watch?v=8wKOGeU2bZg
Jaehwan Lee says:
Dear sir,
I have a question for you.
Let’s suppose if I have tedlar bag sample(it included BTEX gas with 10ppbv(N2 dilution), toluene equivalents). Now I have GC/MS and GC/FI(GC/PID) system to analyze sample. Concentrations as toluene equivalents must be same or not?
This is my questions. I think concentration should be same. Am I right?
I’m looking forward to hearing from you soon.
Jaehwan Lee