When run on a validated method, on an instrument that has properly qualified and certified as good for general use prior to testing unknowns, on a stable instrument, on an instrument that has proper and confirmed Quality Control (QC) immediately prior to use and with an experienced operator with no sampling or preparation error coupled with a person who has the proper credentials to interpret the resulting spectra, Gas Chromatography with Mass Spectrometry (GC-MS) using a single quadrupole configuration is capable of producing a nearly specific result. With all instruments and every assay, there is a limitation. GC-MS is no different.
We have blogged before here our series on GC-MS for lawyers, and just two days ago we also blogged on just such a limitation:
- Introduction-The different configurations and the Electron Impact process
- What types of mass analyzers are there?
- What type of detectors are there?
- What types of analysis can be done?
- How do you read the output?
- How do they come to a qualitative measure using software?
- How do they quantitate the results?
- Do you need chromatography if you are using Mass Spectrometry?
- Toxicology versus solid drug dose examination for controlled substances
With this post, I want to feature another phenomenon that highlights a limitation of GC-MS. There are two well-known and well-studied phenomenon in GC-MS that occurs on occasion: column bleed and septum bleed.
Septum bleed happens when pieces of the septum that protects the injector port makes it all the way through the GC-MS system to the MS detector.
Column bleed is characterized by a noticeably raised baseline that is called by the stationary phase (the inner column coating) coming off and making its way to the detector.
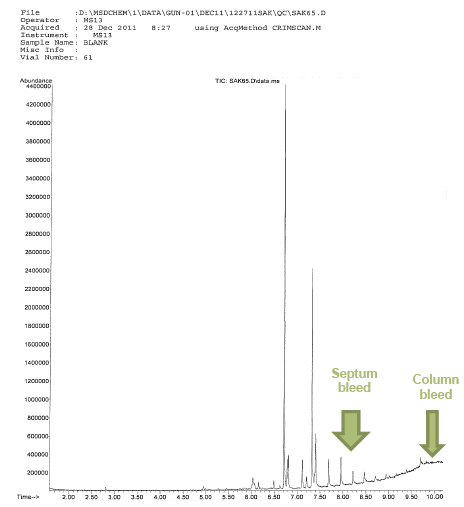
The question is how do we know that it is in fact septum bleed or column bleed? Can’t it be simply a legitimate ion that is the result of fragmentation from the ionizing source?
This can be beyond tricky even for the most experienced expert.
Column bleed is fairly easy to see on the Reconstructed Total Ion Current Chromatogram (TIC) as we can see above. It is featured by an elevated baseline. The issue with this is in the subsequent analysis after the noticeable column bleed event. As we know it is the stationary phase of the column that causes the separation (the resolution) by repeatedly recording column bleed we may very well experience change in the chromatographic resolution. This means that one of the important features of qualitative identification (retention time) may be compromised. Further, when we have high bleed even in one sample, the quantitative validity is suspect in that region of remarkable bleed.
How can we tell when the testifying analyst is incorrectly interpreting true signal versus true column bleed?
Many analysts in the courtroom either out of sheer ignorance, incorrect teaching or downright advocacy will promote and testify that column bleed does or does not matter based upon the particular circumstances that suits the prosecution. Therefore, it is important for us to be able ot tell when this dismissal of the data is truly due to column bleed or is legitimate signal.
What is NOT Normal Column Bleed?
- High baseline at low temperatures
- Discreet Peaks
- Wandering or drifting baseline at any temperature
- Longer columns will exhibit higher bleed than a corresponding shorter column because of the proportionally
- Bleed increases as column diameter increases
- Column bleed is much greater for thicker film columns as there is a greater amount of stationary phase.
Artificial bleed (not due to stationary phase breakdown but is indistinguishable on a TIC) can come about due to contamination. Active compounds such as carboxylic acids, amines, phenols and diols are particularly affected by contamination and can mimic column bleed. It is this carryover (contamnation) that can be easily misinterpreted.
On the other hand septum bleed is due to inappropriate choice in septa, temperature issues (temperature set too high) in the injector or overuse of a particular septum. Agilent and other major manufacturers recommend that the septa be replaced after 35-50 manual injections of 75-100 autosampler injections. Most commonly, on the resulting spectrum we see ions that are 207, 281, 73, 44, 341 (in that order of prevalence).
But what if you have a drug that you are targeting that you are seeking to quantify (so therefore you are running in SIM mode) that uses as a diagnostic ion one of these ions (207, 73, 44, 341)? How can we fairly conclude that the ion is truly diagnostic of our analyte of interest or is due to septum bleed? This is the question.


