There is a large difference between a single column analysis and a dual column analysis when it comes to the ability to most correctly identify and quantitate an unknown in the scientific world.
In forensic science, we are constantly testing unknowns. What is meant by this is that we have a sample that is seized from a crime scene or from a person, but we don’t know what it contains. For example, in blood analysis for EtOH in an alleged DUI case, we have a sample of blood that is taken from the accused, but just by looking at it, we cannot know if there is even ethanol in it, and even if present, how much there is. We need to analyze it using instrumentation in a scientific manner.

When we are looking to be scientific about our analysis we are looking to be as specific as possible, and trying not to be merely selective. There is a large and important scientific difference between being selective and specific. As we wrote before on this blog: Metrology in Quantative Measure: Is it Specific or Selective or Neither…
The International Union of Pure and Applied Chemistry (IUPAC), which is the world authority on chemical nomenclature, terminology, standardized methods for measurement, atomic weights and other critically evaluated data and others have defined the difference between these often confused terms as follows:
A specific reaction or test is one that occurs only with the substance of interest, while a selective reaction or test is one that can occur with other substances but exhibits a degree of preference for the substance of interest. Few reactions are specific, but many “exhibit selectivity”.
Other common definitions include:
Selectivity gives an indication of how strongly the result is affected by other components in the sample.
and also
Selectivity refers to the extent to which the method can be used to determine particular analytes in mixtures or matrices without interferences from other components of similar behavior.
A selective test may be not a specific test due to cross-reactivity, interference, or codetermination.
So, we search for he most specific form of analysis. In the world of DUI for EtOH, the government typically settles for Headspace gas Chromatography with Flame Ionization Detector (HS-GC-FID). GC-FID is not the most specific test available for EtOH examination as there is Gas Chromatography with Mass Spectrometry (GC-MS) for example which is much more selective and borders on specific when it comes to EtOH analysis, but for whatever policy reason, the government chooses not to do the most sceintific thing which is to use the most specific assay available. There is no scientific reason not to test for EtOH on the most specific assay available. In fact, it could be legitimately argued that relying on GC-FID instead of GC-MS for EtOH determination and quantification is not scientific as GC-MS exists and is readily available. However, that is a post for another day.
As we are seemly inexplicably stuck with the scientific step-sister of analysis in GC-FID as opposed to GC-MS, we must look at ways that the government chooses to employ GC-FID to see whether or not as an assay it is valid. As our last series of posts “Method Validation for Lawyers” revealed, there is power in the words “valid” and “validity.” Without having a truly valid method that has been proven to be suitable for its intended purpose, we cannot have a valid result.
Some forensic laboratories choose to use a configuration in GC-FID that is known as a single column, single injection setup. In this set up there is one installed column and the analyst makes one injection (or the autosampler does) to test the sample.

Without any scientific doubt, a single column method of analysis is not forensically or scientifically defensible or acceptable.
Remember that when we use GC-FID, we can never achieve true specificity, the most we can hope for is the possibility of being merely selective as demonstrated and proven through the resolution standard (separation matrix/standard mix). The qualitative result is only based upon one criteria which is the retention time. Retention times through any given column are not unique to one specific volatile organic compound (VOC) to the exclusion of every thing else in the universe. Hence, we have the often repeated phrase that all legitimate technically trained chromatographers know and can recite in their sleep—the limitation of GC-FID is that the retention time is merely characteristic of a compound and certainly not adjudicative or confirmatory of the specificity of that compound— meaning a peak at a given retention time is not a unique qualitative measure (to the exclusion of every other compound in the universe).
Don’t take just my word for it consider the following:
As venerated Professor Harold McNair, PhD writes in his book, Basic Gas Chromatography,
Retention times are characteristic of a GC system, but they are not unique, so GC retention times cannot be used for qualitative confirmation.
He further writes:
Identification of an unknown by comparison to retention times using standards that forms the basis of the qualitative analysis [in GC-FID analysis].
He concludes:
Unfortunately, GC systems cannot confirm the identity or structure of any peak. Retention times are related to partition coefficients (Chapter 3); and while they are characteristic of a well-defined system, they are not unique.
The inferiority of the single column method has been regonized by the Society for Forensic Toxicologists and the American Academy of Forensic Sciences since 2006. The SOFT / AAFS Forensic Laboratory Guidelines – 2006
8.2.4 For ethanol, although false positives are unlikely, confirmation using a second analytical system is encouraged. One approach is to confirm detection of ethanol by GC using an enzymatic assay. Alternatively, confirmation using a second GC column is acceptable IF the second results in significant changes in retention time AND change in elution order of at least some of the common volatiles (e.g. ethanol, isopropanol, acetone). The second analysis should be performed on a separate aliquot of the specimen, or an alternate specimen from the same case.
How do we acknowledge this limitation in the lack of specificity and try to mitigate it?
We can add a different column and analyze the sample concurrently on both columns. As we learned in our post What is a Gas Chromatography column and why should I care?, the column, if properly selected and properly installed, is what primarily causes the separation of the various VOCs to occur. What we do is select two different columns with two different stationary phases. We attach a y-splitter that will take the single injection made into the injector port and divide the sample into two different pathways with one part of the sample going to one column for analysis and the second part going to another column for analysis.

The strength of a well-designed dual column analysis method where the stationary phase is different between the two columns is that this difference in the stationary phase will cause different separation of the analytes (both in terms of retention time and possibly even eluting order) as proven by the chromatograms of the analysis of the resolution standard (separation matrix/standard mix). This different eluting order and different retention times only minimizes, but does not entirely eliminate the possibility of co-elution as again, the resolving (separating) power of the method is only determined by one non-unique measure which is the two retention times. Even though there is a change in the eluting order potentially and the retention times are different based upon the stationary phase composition, again, it must be emphasized that the basic limitation of GC-FID remains in that a retention time is merely characteristic of the analyte, but is certainly not confirmatory.
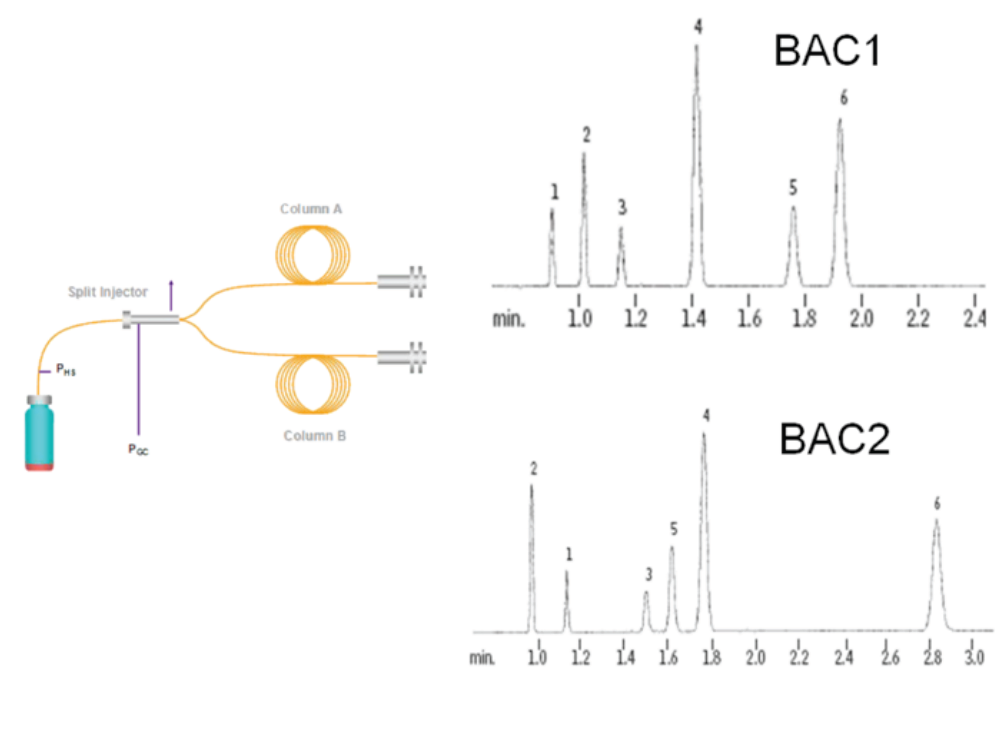
This is why dual column is referred to as the poor man’s Mass Spec as it has a more orthogonal approach towards the qualitative measure than does a single column.
How the current trend of the crime laboratory using a dual column GC-FID is alarmingly unscientific.
What is most alarming to me is the trend that is developing across the United States where the second column is not being used for quantification at all. In this trend that I see sweeping all across the US, the second column is merely being used as a “confirmatory” column in that if the retention time matches with the standards/controls in the Quality Control samples, then it is presented as “verified” in terms of the qualitative measure by simply that second column matching retention time with the knowns that act as the standards/controls. As we explain above, that is a dangerous and unscientific approach.
The reason that this is so important given the above context (there are other reasons that it is alarming, but I want to stick to this reference) is that without the second column giving a quantitative measure, we cannot fairly eliminate co-elution (where two compounds elute at the same time, but only get identified and quantitated as one compound) because if the second column is used to also quantify it will serve as an indirect determination of whether or not there is co-elution. If the second column also provides a quantification of the unknown, we would examine the precision of the quantitative results (how closely the numbers agree among those given by column A and those of column B– do the A’s match the B’s?). If there is co-elution that was “discovered” by the dual column approach, then we would expect to see imprecision between these numbers (the A’s don’t match the B’s).
Now that they have decided as an organization to not quantitate on the second column, we have lost a vital part of quality control and the lab supervisor has lost a powerful tool of quality assurance. It is just bad science.
Further, there is no legitimate scientific reason for not quantitating on the second column. If you are doing good quality work and your methods and instruments are in control, then your numbers should agree.
It’s not a time thing.
As it is a single injection y-splitter dual column analysis anyway, the amount of time it takes to make a calibration curve, evaluate it and then incorporate it into the software for one column is virtually the same amount of time to do the same on column 2. There is virtually no added time. It makes no sense from a science point-of-view.
So in conclusion, we can fairly conclude the following:
- Scientifically, we always want to test our unknowns on the most specific assay available.
- GC-FID is not the most specific assay available.
- Laboratories that use single column GC-FID as their method of analysis do not produce forensically or scientifically defensible or acceptable results.
- Laboratories who use dual column GC-FID that do not quantitate on both their columns do not produce forensically or scientifically defensible or acceptable results.
- Good science is not always practiced in the modern state crime laboratory.


