Co-elution is a very important concern in forensic chromatography. In a nutshell co-elution can be defined as when non-resolved (non-separated) compounds elute (come off) the column at the same time. It comes comes in two forms: (1) lack of specificity (inter-sample co-elution), and (2) analytical carry-over (intra-sample co-elution).
Lack of specificity:
A Gas chromatograph is a dumb machine. Out of the box, it knows nothing. It has to be “taught.” There is a specific way that we have to teach it. This process of how we teach it is called quality control. We have to teach it both how to render a qualitative result (what do we have?) and a quantitative result (how much do we have?). We have to teach it what our target analyte is (e.g., EtOH), but most importantly what is not our target analyte through the process of proving resolution (separation) of like analytes that may organically be in the sample of our matrix (e.g., the blood). Once it is properly taught what is not our target analyte, then we can say that the method is specific if and only if it can truly pick out our target analyte to the exclusion of every other analyte in the universe. Otherwise if the method can only tell the difference between only a handful of analytes, we can only conclude the qualitative measurement is characteristic of the target analyte as there is a possibility of co-elution. Two separate compounds are not distinguished from one another. They are not separate.. Due to this minimal amount of resolving power (distinguishing power), we only call the method merely selective and certainly not specific.
For example, in the case of EtOH analysis in blood, our target analyte would be EtOH, and the like analytes of what we need to “teach” the machine what is not EtOH by proving good resolution (separation) from other volatile organic compounds include, at a minimum, the following compounds:
- methanol,
- isopropanol,
- butanol,
- n-propanol (which is likely our internal standard),
- acetone,
- acetonitrile,
- acetaldehyde,
- toluene,
- Trihalomethane,
- chloroform and other chlorinated hydrocarbons,
- Tetrachloroethene,
- methyl-tert-butyl,
- Benzene,
- styrene,
- Ethylbenzene, and
- methyl ethyl ketone
This type of co-elution is due to the inability of the method to resolve (separate or distinguish) compounds within the same sample. In simpler terms, they are stuck together.Whenever I think of this type of co-elution, I think of this movie:
How can you tell when it’s there:
Sometimes, it is easy such as:
- a doublet (two peaks at the top unresolved down to the baseline)
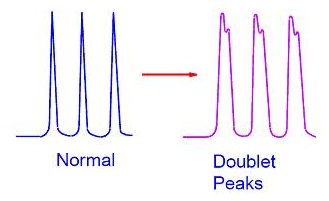
- peak tailing
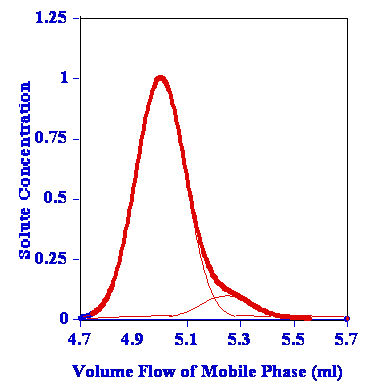
- The worst type is not when there is a doublet, peak tailing or two clearly unresolved peaks (BV to VB in the data table), but when the one peak totally subsumes the other.
Due to all of the above, I call intra-sample co-elution because the issue of co-elution comes all from within one test and no exterior sources.
Carry-over and contamination:
When we use standards/controls (if they are at the beginning of the run we call them calibrators; if they are in the middle or the end, we call them verifiers), for best modern forensic science practices, all standards/control should come from Certified Reference Materials (CRMs).
- A Certified Reference Material (CRM) is an independently produced material that has been rigorously tested per ISO Guide 35: 1989 and has been found to have only the target analytes listed on the certificate of analysis (COA). (Read more here- https://www.thetruthaboutforensicscience.com/reference-materials-and-standards/) There are many third party vendors such as Cerilliant, Restek, Sigma Aldridge and others who make these materials. There is, of course, the National Institutes of Standards and Technology (NIST) who makes their own brand for commercial sale that they call Standard Reference Materials (SRMs).
This is to be contrasted with “home brew” standards/controls where the laboratory itself decides to make its own. It is a minor money saving initiative. But the risk/benefit analysis of getting it wrong/saving money, in my mind, is unacceptable. The problem with the home brew method is really 3 fold:
- There is not a crime laboratory in this world that has quality control or quality assurance in place that will meet the ISO Guide 35 standards. So, by definition, their home brew efforts will be of inferior type and kind.
- Quality of raw materials. The usefulness of the home brew is limited by the quality of raw materials used. Did the laboratory use USP grade raw materials and deionized, denatured water? We don’t typically know. Very few (read as none that I know of) actually check through their own analysis the batch of USP grade materials that goes into their home brew before they deploy it as the raw material (source material) that they make their standards from. You always test before you deploy. Basic science. In science, you can’t just trust the other guy. You have to independently prove the other guy is right before you use it. But hey, why be scientific we’re in a state crime laboratory, right?
- Contamination. Think about it. Crime laboratories are full of under-trained, undereducated people (who may or may not mean well) whose results and proficiency are rarely rigorously examined by anyone, operating under extreme pressure in terms of throughput (test more samples faster) who typically don’t understand quality assurance culminating in the perfect storm of cutting corners or unintentionally making mistakes with no real oversight (inferior quality assurance) to catch it. The training into contamination control often consists on a focus on biological material (don’t get HIV or Hep C) and how to not accidentally get high while testing the cocaine as opposed to focusing on best practices of Pipetting, cleaning of instruments, cleaning of volumetric flasks, and being aware of how easily Volatile Organic Compounds (VOCs) transfer.
All of this leads to the second to worst laboratory analytical chemistry disaster in my mind called carry-over (the worst is analyzing the wrong sample and reporting it out as if it were the other sample)
- Carry-Over: A process by which materials are carried into a reaction mixture to which they do not belong. It can be either unidirectional or bidirectional in a series of specimens or assays. The term carry-over effect is used for carry-over from specimen to specimen.
There is an old chroamtography saying that goes: “Just because you are done with the chromatography, the chromatography is not done with you.” What this means is that even though you artifically set the termination of the test for a particular injection to be some time (e.g., 3 minutes, 8 minutes, then you inject another sample) does not mean that all of the compounds have fully eluted from the injection that you made as there may be retained compounds that elute after the artificially end of the test. As the analysis goes longer in time (vial or cycle numbers increase), we see unidentified peaks in the analysis of the CRMs. As discussed above, CRMs are pure. They have been adjudicated and proven by others to have nothing other than the targeted analytes which means each peak must be identified.
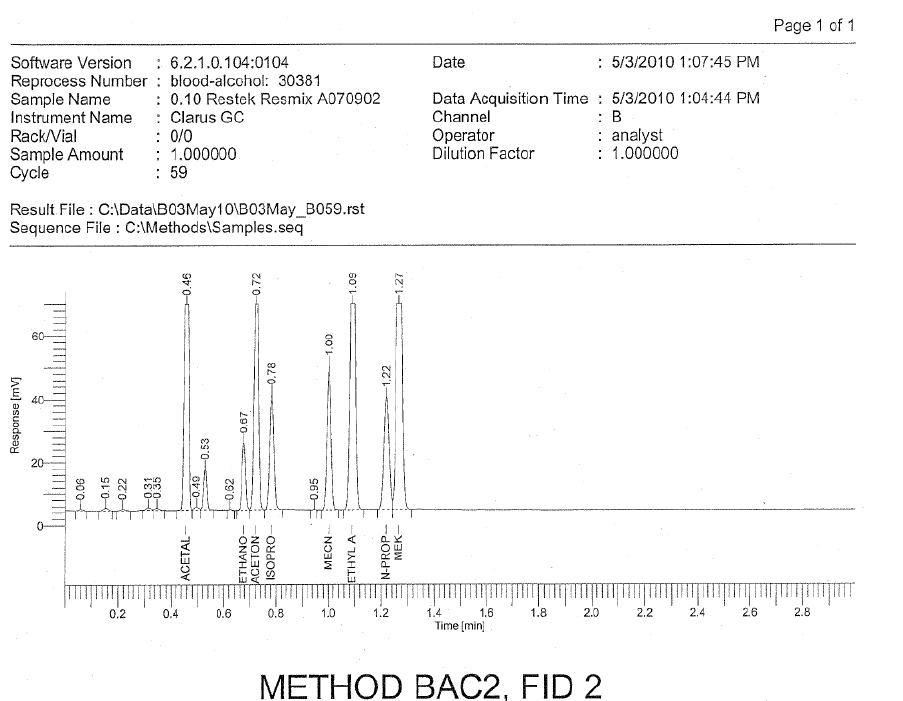
If there are unidentified peaks such as the case here in the CRMs, then we can fairly and scientifically conclude that there is analytical carry-over. This can be inferred, but not proved, by the use of home brew with the issues 1 through 3 above.
The problem with carry-over is that if it all of the stars line up correctly, we can have analytical carry-over from a longer retained compound from a previous injection that elutes at the same retention time as that which is characteristic of EtOH of our current injection.
This type of co-elution is due to method design. In simpler terms, they are on top of one another. Whenever I think of this type of co-elution, I think of this movie:
Due to all of the above, I call this inter-sample co-elution because the issue of co-elution comes from outside of this one test and from an exterior source.
Conclusion:
The machine can’t tell what part of the peak comes from the carry-over or an unresolved compound that is not EtOH that comes from the previous injection or from a lack of specificity versus what is the accused’s fault because he/she imbibed EtOH. It counts anything and everything that elutes (comes out) at that time window (rentention time) as EtOH.
_____
Citations:
David L. Ashley, Michael A. Bonin, Frederick L. Cardinali, Joan M. McCraw, and Joe V. Wooten, “Measurement of volatile organic compounds in human blood.” Environ Health Perspect. 1996 October; 104(Suppl 5): 871–877
Brugnone F, Perbellini L, Faccini GB, Pasini F, Maranelli G, Romeo L, Gobbi M, Zedde A. Breath and blood levels of benzene, toluene, cumene and styrene in non-occupational exposure. Int Arch Occup Environ Health. 1989;61(5):303–311.
Cammann K, Hübner K. Trihalomethane concentrations in swimmers’ and bath attendants’ blood and urine after swimming or working in indoor swimming pools. Arch Environ Health. 1995 Jan-Feb;50(1):61–65
Popp W, Müller G, Baltes-Schmitz B, Wehner B, Vahrenholz C, Schmieding W, Benninghoff M, Norpoth K. Concentrations of tetrachloroethene in blood and trichloroacetic acid in urine in workers and neighbours of dry-cleaning shops. Int Arch Occup Environ Health. 1992;63(6):393–395
Brugnone F, Gobbi M, Ayyad K, Giuliari C, Cerpelloni M, Perbellini L. Blood toluene as a biological index of environmental toluene exposure in the “normal” population and in occupationally exposed workers immediately after exposure and 16 hours later. Int Arch Occup Environ Health. 1995;66(6):421–425
Gill R, Hatchett SE, Osselton MD, Wilson HK, Ramsey JD. Sample handling and storage for the quantitative analysis of volatile compounds in blood: the determination of toluene by headspace gas chromatography. J Anal Toxicol. 1988 May–Jun;12(3):141–146
Ramsey JD, Flanagan RJ. Detection and identification of volatile organic compounds in blood by headspace gas chromatography as an aid to the diagnosis of solvent abuse. J Chromatogr. 1982 May 21;240(2):423–444
Ashley DL, Bonin MA, Cardinali FL, McCraw JM, Wooten JV. Blood concentrations of volatile organic compounds in a nonoccupationally exposed US population and in groups with suspected exposure. Clin Chem. 1994 Jul;40(7 Pt 2):1401–1404
Wallace L, Pellizzari E, Hartwell TD, Perritt R, Ziegenfus R. Exposures to benzene and other volatile compounds from active and passive smoking. Arch Environ Health. 1987 Sep-Oct;42(5):272–279



