False Conclusions and False Convictions: Attempts of Elucidating Pharmacodynamic Effect from an Analytical Chemistry Result-How Solely an Analytical Chemistry Result in a DUID Prosecution Cannot Scientifically Support a Conclusion of Driving Under the Influence of Drugs
To download this post as a finished pdf article please click on the below:
False Conclusions and False Convictions- Attempts of Elucidating Pharmacodynamic Effect from an Analytical Chemistry Result.pdf
By: Justin J. McShane, Esquire[i]-Harrisburg, PA
Abstract: No one should be permitted to opine pharmacodynamic effects of a multiple order kinetic drug based upon an analytical chemistry result alone.
This is because of the very interesting world of pharmacokinetics and the multi-variant problem of the human body and the human condition. The analytical chemistry result must be equated not simply to symptomatology, but uniquely to it, meaning that the use of the drug uniquely and to the exclusion of all other reasons produced the observations of the marked diminution of dexterity, marked diminution of cognitive function or marked diminution of psychomotor function.
Discussion:
What are the numbers really telling us?
Across the United States this question is repeated asked several times each day. Frequently, the answer is wrong, even from experts.
In a typical scenario, blood is taken one hour to two hours after the motor vehicle stop and reveals a “magic number” of 70 ng/mL of Alprazolam (Xanax). No alcohol or illegal drugs were found and the motorist had a valid prescription for Xanax. The officer now has this analytical chemistry result, but the ultimate question of impairment is not that easy to devine.
If you knew nothing about the world of pharmacology and you were presented with a seemly large number such as our example from the analytical chemist examining the drug, then you may think this was a Driving Under the Influence of Drug (DUID) case.
But is it necessarily so?
Is this a fair conclusion based upon this data?
There are limitations to analytical chemistry and what it can tell us.
Analytical chemistry is the constellation of preparative and instrumentation steps of an assay (testing method) that results in a reported qualitative measure of the drug and a quantitation of only that drug in the body at the time of the blood collection. Analytical chemistry inherently does not take into consideration pharmacodynamics. It is the end result of a process that depends upon the input it is given. Blood draws do not happen concurrently with driving and therefore at most it is a measure of the drug’s presence and amount in the blood at the time of the blood draw and not reflective of the time of driving. Stated differently and more simply, the human body itself is unique to its individual owner and variations can impact the value of the analytical chemistry result and its later interpretation as to the drug’s effect to this unique human being (pharmacodynamics) that is the citizen accused.
Is the 70ng/ml level low, high – inside or outside therapeutic range?
Is this value likely to produce significant impairment?
Did the combination of this drug plus others taken from over-the-counter sources produce a synergistic (additive) or antagonistic effect?
Well, analytical chemistry alone cannot answer these questions relevant to impairment for us. Each person is different. Mama was right. You are like a snowflake. You are unique pharmacologically and respond to the effects of a drug in a unique pharmacodynamic manner. From a strict analytical chemistry to pharmacology point-of-view, having a “magic number” alone cannot prove impairment in a DUI case.
This is where pharmacology comes into play. However, there are limitations and conditions precedent even to pharmacology.
Even a trained and clinically experienced pharmacologist has limitations of his or her interpretation of the analytical chemistry result as related to pharmacodynamics. The very minimum information that would need to be known in order for a trained pharmacologist to begin to consider determining the possibility of impairment includes the following:
1. In the case that the police officer made observations of dexterity difficulties, cognitive function issues or psychomotor function dysfunction, or even if he or she attempted to perform or completed a DRE evaluation, the officer or later expert must have information that the motorist is “normal,” meaning that the person was free from any medical pre-conditions that could be confused for impairment. As it is the basic assumption of any observation that there is a noticeable change from the person’s homeostasis, there must first be a known and established homeostasis to establish deviation. One cannot fairly assume that the person is “normal” and dexterous or cognitively quick or psychomotor coordinated. There are many people in this world who are not.
2. The officer or later expert must be privy to the person’s pre-existing physical or mental conditions and then must rule any and all of them as possible contributors to the perceived observations that are later interpreted as impairment.
3. The officer or later expert must be privy as to what symptoms or diagnosis originally lead the doctor to prescribe the medicine to begin with so as to be aware of the person’s un-medicated state. It is an assumption that without the drug that the person would not be impaired. Therefore this data of the person’s un-medicated state is necessary to rule out the possibility that the perceived dexterity difficulties, the cognitive function issues or the psychomotor function dysfunction were due to an inappropriately low dosing and therefore that the person appeared impaired when they were not.
4. The officer or later expert must be aware of all medications that the person ingested including the over-the-counter ones so as to be clear that the affect of the analyte of interest and the other medications either over-the-counter or controlled did or did not influence the measured drug in terms of impairment.
5. The officer or later expert must be aware of the dosing history of the patient in terms of the supposed impairing drug as the dosing history may profoundly impact the affect of the drug dose on the human.
6. The officer or later expert must be aware of the recent dosing usage of the patient as it too may impact the conclusion of impairment.
The myth of the one-size fits all “therapeutic range”
As we can quickly see, the concept of a number-based “therapeutic range” of normal and high is not a valid pharmacological model. It is a convention and a device that is ripe for abuse by the under-trained and under-educated. It is at best a tool to begin to determine pharmacodynamic effect, but is certainly not conclusive.
The primary purpose of the therapeutic drug level tables is clinical in nature, not forensic. They are to be used to adjust the dosage of a patient into a range that has been shown to be therapeutically effective for a group of experimental subjects. Clinically this is done by taking a blood sample immediately prior to the next dose after obtaining steady-state. The patient’s dose would be adjusted either up or down based upon the plasma or serum level (not whole blood). Toxic levels which are often part of such a table are based upon adverse side-effects that have occurred and blood levels determined. The important point is that these levels have not been correlated with any behavioral effects (pharmacodynamics). There are some drugs that have been studied for their behavioral effects and correlated with plasma levels but these are not in published tables. There are tables of drug levels associated with deaths and these are regular used by medical examiners as but one of many possible factors to help assign a cause of death based upon the totality of the circumstances, but they are not used alone to determine cause of death.
Too many factors need to be considered, such as:
Was it a single-dose event?
Is the person on a maintenance program (e.g., Methadone, Xanax and Lorazepam)?
Was the person in the absorptive phase, peak or elimination phase at the time of the measure?
Was the person in the absorptive phase, peak or elimination phase at the time of the driving?
What was the potential impact of the drug on the motorist in terms of dexterity, cognitive function or psychomotor function?
Is the analytical chemistry result at the time of the blood draw even relevant at all?
Most importantly, the complicated question of retrograde extrapolation[ii] is even trickier in the case of multiple order kinetic drugs.
Most undereducated “experts” who testify before the Courts have made the assumption that most therapeutic drugs are eliminated by first order kinetics alone. In fact, many therapeutic drugs are eliminated by second and even third-order kinetics. Some highly lipid soluble drugs like THC is fifth order kinetics. The important point is that the terminal phase of elimination can be described by first order kinetics. With only one point on the elimination curve we have no idea where the value fell.
For the sake of further discussion, let us assume that a drug has second-order kinetics and one point is taken. We are not sure whether it falls on the first slope (that is usually continuation of distribution) or the second slope, which is the terminal phase of elimination.
This is why the best practice, if one wanted to be sure would be to take no less than eight (8) serial draws of a person’s blood over an extended period of time to have enough data to fairly determine based upon data where the person is on the dose-response curve, how they are clearing the drug from their body, and all the while make concurrent observations of that same person’s physical, cognitive and psychomotor function at those times.
The difficulty of opining with simply one data point of the provided analytical chemistry result at the time of blood draw back to the true analytical chemistry measure and the true pharmacodynamic impact at the relevant time of conduct (i.e., driving) concerns the interplay of pharmacokinetics of drugs that are not zero order kinetics as is true with EtOH. The pharmacokinetics of drugs other than EtOH involves a very complicated internal process of the body. Pharmacologists spend many years studying pharmacokinetics[iii] and dose-response. This issue of half-life and the steady state truly illustrate the danger of relying upon the assumption inherent in the quick reference charts that a certain measured amount of a drug is beyond the therapeutic range and therefore impairing.
Further complicating the issue is the idea of pharmacogenetics. There is a wide spectrum of response in terms of dose and response that can be of genetic, physiological, pathophysiological, or environmental origin. Also there is an additional dimension that there can be a wide spectrum of drug concentration in the blood after application of the same dosage between people. Pharmacokinetic and pharmacodynamic variations can appear at the level of drug metabolizing enzymes (e.g., the cytochrome P450 system), drug transporters, drug targets or other biomarker genes. Pharmacogenetics is a very relevant forensic consideration in both pharmacokinetics and pharmacodynamics[iv].
The myth that based upon the analytical chemistry result we can fairly conclude that someone “took one too many”
It is simply scientifically and pharmacologically irresponsible to conclude that simply and solely because of an analytical chemistry result that the person must have “taken too many” or went beyond the prescription.
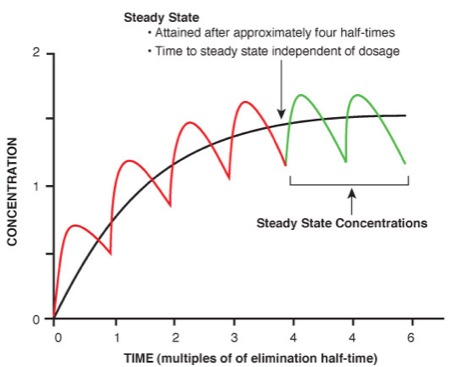
When the patient is taking a medication on a regular basis, there is an ongoing process of drug absorption in the form of each dose of the drug and, concurrently, an ongoing process of drug elimination. Eventually, there comes a point when the amount of drug going in is the same as the amount of drug getting taken out. This is the “steady state.” It takes somewhere between 5 and 6 half-lives for a medication to reach steady state. Thus, medications with short half-lives reach steady state relatively quickly, while those with long half-lives take a long time to reach steady state.
When the drug reaches steady state, these effects can be either attenuated or completely absent. Unwanted side effects, such as impairment, from a particular compound are great deal more acceptable if they only take place on the way to steady state (i.e., they are transient).
However, as is frequently the case in DUID prosecutions where we simply have a single number that provides us with the amount of the drug in the blood at the time of the blood draw (N.B., not at the time of driving), how do we know if we have not achieved steady state or how do we know we are on the way to steady state? More importantly with a single number and without the benefit of the clinical notes and observations of the prescribing physician how do we know that the analytical measure does not represent successful achievement of the steady state?
If we only have one data point on a graph, how can we ever honestly elucidate these important questions?
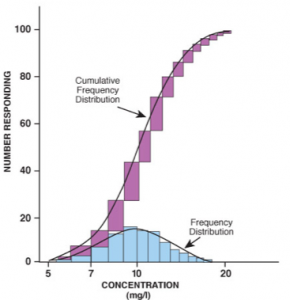
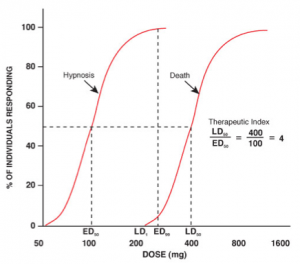
As one can see by the above graphs[v], it is not possible to predict where an individual person is on the dose-response curve without serial testing.
Notice on the top graph, that the therapeutic amount of a specific drug may vary four-fold universally across the population.
Now notice on the bottom graph, that a “therapeutic” amount for a particular person may represent a “lethal” amount for another person.
With the exception of EtOH, all other drugs are not zero order kinetics and interdependent of the concentration of the reactant. The rate equation of a reaction within a multi-step system cannot be deduced from the stoichiometric coefficients of the overall response as a whole; therefore, retrograde extrapolation is nearly impossible or fraught with peril.
Remember, it takes 6 half-lives to eliminate 97% of the analyte of interest in the system. Add to that the issue of chronic dosing or re-uptake perhaps not in the uniform times necessary in order to achieve “steady state”.
Some folks, when dosed with the same amount of the same drug will react entirely differently than others. The fact that blood drug-levels alone, meaning in the absence of serial draws or other pharmacologically relevant data, cannot predict a pharmacodynamic effect has been known for decades. The combination of the kinetics involved and the genetics involved in a unique human being provide that making a pharmacodynamic prediction or opinion based upon incomplete data at best is a wild guess. The most logical and scientifically valid conclusion when all or significant parts of the pharmacological data is missing is to render an opinion that the effect is inconclusive based upon the known information. To do otherwise would invite error.
In the clinical world which is a controlled environment where the dosing history and dosing effects of patients are tracked and known over time and where more than one blood draw is taken, a clinician with full knowledge of all of the factors outlined above may be able to conduct therapeutic monitoring of drugs. The prime examples of this legitimate use of analytical chemistry in conjunction with pharmacological variables that are known exist in the use of anti-epileptics, antibiotics, digoxin, cyclosporine and many more drugs.
Again, if dealing strictly on the pharmacological level in terms of a conclusion of impairment without a context or a baseline of comparison, it is not scientifically or pharmacologically responsible to conclude impairment based exclusively upon the analytical chemistry result. This is especially so with the presence of SSRI’s (selective serotonin reuptake inhibitors or serotonin-specific reuptake inhibitors). SSRI’s are found in antidepressants used to treat depression, anxiety disorders, some personality disorders and occasionally, insomnia.
Conclusion
There is a limitation to analytical chemistry. The idea of simply and solely using an analytical chemistry result and being able to determine impairment is a dangerous suggestion. The idea of interpreting the analytical chemistry result with an eye towards opining impairment is a very complicated task that should be reserved to highly trained pharmacologists who have years of clinical experience with that particular drug that is hypothesized in this case to cause impairment and only then with complete and total relevant clinical data to that unique person. Without knowing all of the necessary pre-requisites outlined above, meaningful retrograde extrapolation to the relevant time (i.e. the time of driving) and then later predicting pharmacodynamic affect at that time, is at best guesswork and at worst a fool’s errand. This number produced (i.e., the analytical chemistry result) without the entire relevant rich clinical context truly has the overall significance of random numbers chosen in a lottery drawing. Essential to this exercise is the need to connect the apparent physical, cognitive and psychomotor manifestations directly and most importantly uniquely to impairment as opposed to homeostasis, which most government experts or roadside officers cannot do based upon the limited data that is available to them. Even the “magic” and pseudo-science of the DRE evaluations recognize this basic flaw and limitation, especially with respect to poly-substance use.
Bottom line: Each person is different. Mama was right. You are like a snowflake. You are unique pharmacologically. From a strict analytical chemistry point-of-view as then later interpreted to conclude pharmacologic affect, having a ‘magic number’ alone cannot prove impairment in a DUI case.
[i] Justin J. McShane, Esquire is a Harrisburg, Pennsylvania-based attorney who specializes in using forensic science for the benefit of the citizen among us who has been accused. He is a Board Certified trial attorney by the National board of Trial Advocacy as well as Board certified as a Dui Specialist per the National College for DUI Defense, Inc. He is a Fellow with the American Institute of Chemists (AIC). He is a member of numerous scientific organizations including the American Chemical Society (ACS), American Institute of Chemists (AIC), American Academy of Forensic Science (AAFS), American College of Forensic Examiners, Society for Analytical Chemists (SACP), National Fire Protection Association (NFPA), The Chromatography Forum of Delaware Valley, American Society for Testing and Materials (ASTM), American Association for Clinical Chemistry (AACC), National Conference of Standards Laboratories International (NCSLI International), Association of Analytical Communities (AOAC International), American Society for Mass Spectrometry (ASMS), Society for Applied Spectroscopy (SAS) and the American Society for Quality (ASQ). He is a published author. He is a frequently invited guest lecturer at national, state and local seminars that are attended by lawyers, judges, scientists and policy-makers. He has twice been invited to lecture at the ACS National meeting and has been accepted to present AAFS. He is the Chairman/CEO of The McShane Firm, LLC, a six attorney criminal defense and DUI law firm. He maintains two blogs: www.TheTruthAboutForensicScience.com and www.PADUIBlog.com
[ii] This is the scientific term for the ability to take someone’s analytical chemistry result at the time of blood draw, and use a defined mathematical formula to extrapolate (look backwards) to determine what the level was at the time of driving. It requires proof that the person is in the post-adsorptive stage along with several other assumptions that typically the police officer does not have.
[iii] First order kinetics is defined as drugs where the rate of elimination is proportional to the amount of drug remaining in the body. The majority of drugs are eliminated in this way during their terminal phase of elimination. A constant fraction of the drug in the body is eliminated per unit time.
[iv] Pharmacogenetics and forensic toxicology, Forensic Science International, Volume 203, Issue 1, Pages 53-62, 15 December 2010
[v]Goodman and Gilman’s The Pharmacological Basis for Therapeutics



Angela says:
I have a question and feel like i get alot of run around here in the state of TN. MY son was pulled over and is being tryed for dui which took the courts from jan to nov to get his blood work back. It came back 1.29ml of xanax and 165 ng of thc and no one can tell me if this is over the limit and if its a dui not even the PD. we go to trial now feb13 and guess im just wondering if you would know or know someone here in the tri citiea area that can help me know if it falls under a dui?
Angela says:
Sorry i should be more detailed we are in kingsport TN,tri cities includes kingsport,bristol and johnson city Tn