In a series of posts, we are going to talk about Mass Spectrometry.
- Introduction-The different configurations and the Electron Impact process
- What types of mass analyzers are there?
- What type of detectors are there?
- What types of analysis can be done?
- How do you read the output?
- How do they come to a qualitative measure using software?
- How do they quantitate the results?
- Do you need chromatography if you are using Mass Spectrometry?
- Other topics of interest about GC-MS
There seems to be a debate, more like a scientific war, between spectroscopists and chromatographers. It boils down to this fundamental question:
Does co-elution matter if one uses Mass Spectrometry?
Well, the answer is yes, of course. Here is why…
- If it were indeed true that we do not need well resolved (separated) and specific peaks before we use mass spectrometry (i.e., co-eluting peaks don’t matter), then we would not waste our time with the chromatography aspect of the gas chromatograph. The GC part of the GC-MS method takes the most amount of time using this technique, and if we could cut that out completely and perform instead what is called a direct introduction (DI) or direct interface (DI) probe into the MS alone, then we would increase throughput tremendously. We could test so many more samples. But we don’t and for good reason. One of the reasons that we just do not perform DI and we need chromatography with need well resolved peaks is that it is very easy to use too much sample in the DI method system.

DI to the MS - If you are testing pure compounds, then DI may be a very useful technique. It is fast and rugged requiring very low sample size. However, in our world, the forensic world, the chances of either getting a pre-consumption unknown drug in pure form is extremely rare. Further if the sample is in post-consumption matrix (e.g., blood, tears, sweat, urine, blood), then we know that the sample is not pure.
- Professors Harold McNair, PhD and Fred W. McLafferty, PhD as well as Dr. Marvin C. McMaster and Dr. Lee Polite warn against introducing into MS analysis anything other than pure compounds (one way to get pure compounds is through the use of a GC). Professor McLafferty in Interpretation of Mass Spectra wrote as follows: “If several compounds are present in the sample, the resulting spectrum will represent a linear superposition of the competent spectra.” Dr. McNair puts even more simply, “Just don’t do it. Use the advantages of good chromatography first, then you have little chance of error in the reporting of your results.” Another authority in the field Dr. Marvin C. McMaster writes “A mass spectrometer is an excellent tool for clearly identifying the structure of a single compound, but it is less useful when presented with a mixture.” He further writes “A good chromatographic separation based on correct selection of injector type and throat material, column support, carrier gas and oven temperature ramping, and a properly designed interface feeding into the ion source can make or break the mass spectrometric analysis.” He concludes, “The mass spectrometer is designed to analyze only very clean materials.” Another noted international instructor for Agilent, Dr. Lee Polite, PhD, MBA writes, “If you want to be sure and you are in the business of being sure, then separation first always before MS work.”
- The other issue is human integrity. While there are a lot of analysts who have high standards for themselves. Some really care about what they do and want high quality of their results. However, there are some that do not share that vision or care. In the worst case, there is fraud. The issue of co-elution of the GC into the MS invites issues surrounding human integrity.
It is a question of could versus should. Could you perform MS without GC or use GC in a way that doesn’t resolve peaks and not prove for a purified compound into the MS for analysis? Sure. You clearly can. BUT, will you be right in your result? Possibly not. Clearly best practices would be to use the powerful tool of GC as it is intended and as it is designed which is to provide for purity and specificity in the effluent. Why would you invite or promote the possibility of error if you did not have to? Why would you invite or promote the need for human integrity. Why if it is not necessary???
As the video above shows us, there is always clearly a”human factor” in all of this analysis. In fact, there is a lot! To a degree, we are left to the discretion of a human being. Scary.
The reporting that is provided is just a small sliver of what can be provided to reviewing individuals. For example, what reviewing counsel and experts typically get are a one sheet conclusion piece of paper.
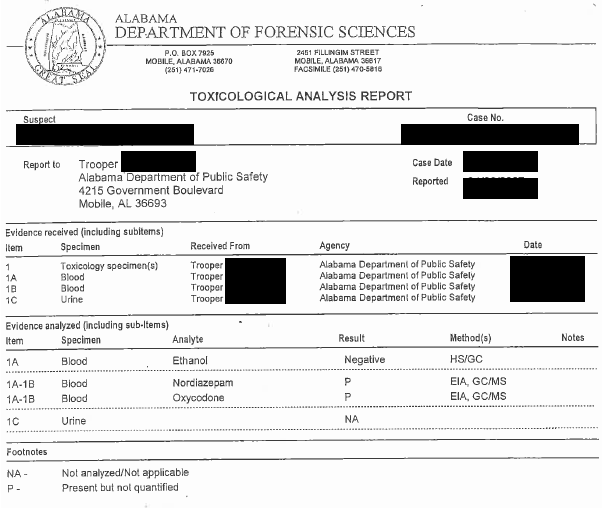
Here is a typical conclusory report that a defense attorney may get. As you can see no detail, just a conclusion.
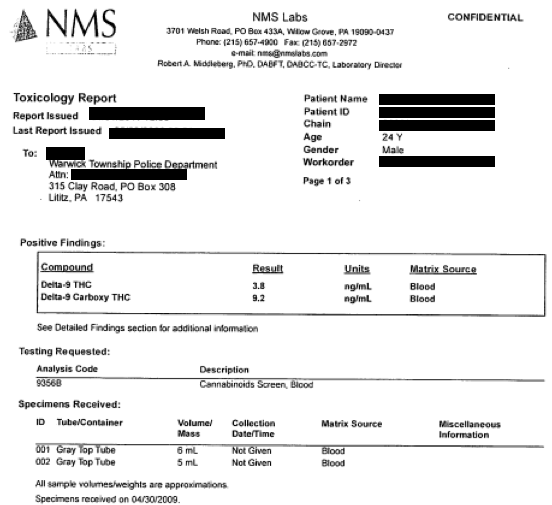
Here is a typical auto-report from GC-MSD Agilent software. Again, not a lot of detail is provided.
But we can get a lot more information from the GC-MSD software such as these from Agilent:
- The full size TIC printout, and
- Method printout
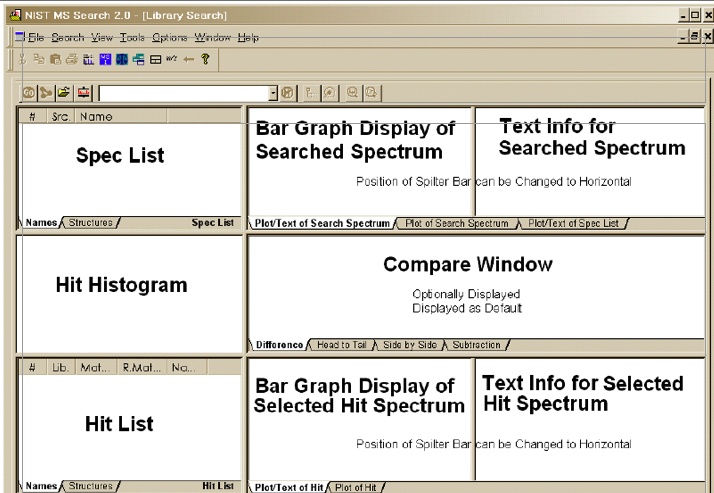
And we can get significantly more information from the NIST search software simply by right clicking on the screen below such as these reports:
- The full information printout on anywhere between the top 1 to 100 results from the NIST spectral library. (This one is the top 10.) There are various click boxes where you can add or subtract information;
- The full unknown mass spectrum from the display “Bar Graph Display of Searched Spectrum;”
- The full information from the display “Text Info for Searched Spectrum;”
- The full information from the display “Text Info for Selected Hit Spectrum;” and
- The full information from the display fields “Compare Window” including “Subtract,” “Side by Side,” “Head to Tail,” and “Difference.”
- Perhaps the most important is the Hit List with the Library identified, the Match Factor, Relative Match Factor and the Probability Score.