The top 2 problems seen with Gas Chromatography BAC results
The testing method used most frequently in blood based Blood Alcohol Content (BAC) analysis in the United States is called headspace gas chromatography (GC) with flame ionization detector, using wall coated open tubular capillary columns. The process analyzes chemicals without decomposing them.
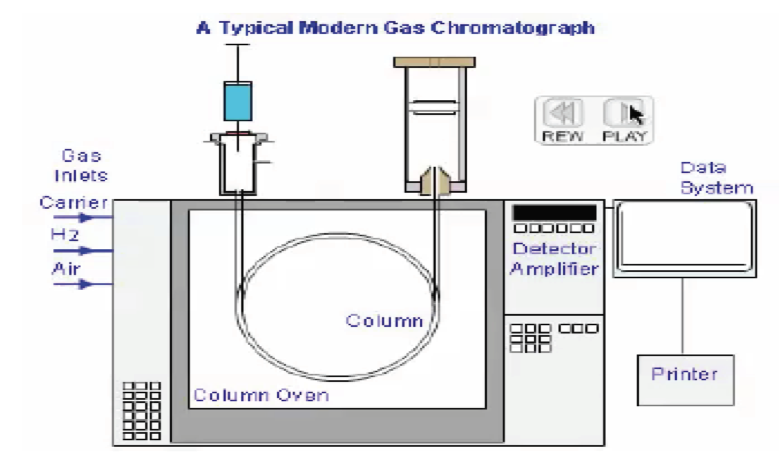
The GC is a complex machine that has many variables. However, if used properly, the procedure can take a mixed up sample (such as blood) and provide for unique and specific separation of a certain compound (such as ethanol) and then also correctly quantify the substance tested (the numerical value expressed as a BAC). A valid BAC can only come about if, and only if, there is proof that (1) there was a properly configured machine, (2) there was proper quality control (such as the use of ethanol free blanks that are blank of ethanol), (3) there was sufficient separation (proper targeting of ethanol alone), and (4) the machine was properly calibrated to give a correct number.
In essence to have a valid result, it is a twofold process. It is a conjunctive and not a disjunctive. Meaning that in order for there to be a valid test, both key elements must be proven, and not simply presumed. GC’s goal is to uniquely identify a compound, one specific chemical compound to the exclusion of all other chemical compounds, in qualitative analysis and then determine the amount of the compound that is present in a quantitative analysis.
It is this first step (proof of separation and proper targeting of only ethanol) that is vitally important to arrive at a possibly valid result. It is where I see a lot of errors in forensic crime laboratories. It is either poor proof of separation, a lack of meaningful separation or no proof of separation that is the problem.
As all properly trained analytical chemists would agree: “If you don’t have separation, then you can’t move on to quantification reliability.” Put simply, if there is no proof of separation and unique proper separation, then there is no valid test. The number (the BAC) that results must be due from ethanol and nothing else combined with it. Without this proof, “we fail” and “the test is no good” because there is no valid BAC.
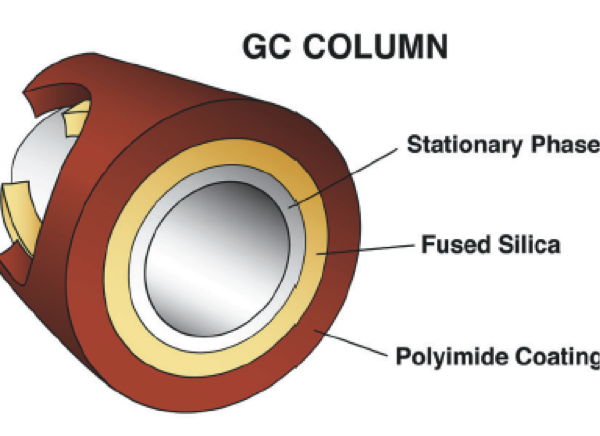
Technically, separation is primarily either created or not created by the GC’s column. A column (the heart of the chromatograph) is essentially a hollow tube. Its inside is coated with various substances (the stationary phase) that attracts different compounds differently (affinity). If the compound is attracted to the walls where the coating is, then it is slowed down its path to the end when compared to its cohorts that enter the column at the same time. Generally, a given column is supposed to separate the chemicals depending on the column’s characteristics and the chromatograph’s particular configuration. There is no universal column. There are many. The choice of column determines whether or not we can separate out ethanol by itself or if the result (the BAC) is really ethanol combined with something or “somethings” else on top of the ethanol. This combination effect is called co-elution and over reports a BAC. This is why proof of separation is so essential to provide for a valid result. Without it we cannot trust the BAC.
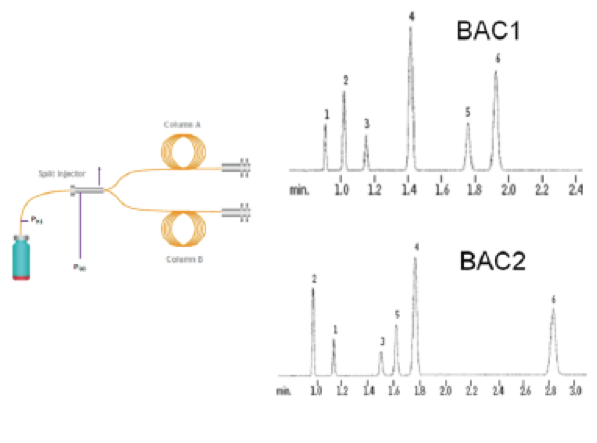
In a well designed and validated method, as the sample (the headspace above the blood) that has a lot of chemical compounds mixed in it goes through the machine, the different chemicals get attracted to the walls of the column differently and hence travel at a different speed through the column. If configured properly, the machine through the column causes the compound, in this case ethanol, to elute (move), at different rates (measured in minutes) than every other chemical. This timing is known as retention times (Rt). If done properly, the machine analyzes the Rt to provide the analytical result based upon a combination of: (1) Rt (the qualitative result-what do we have?) and the peak area at that Rt (the quantitative result-how much do we have of that?).
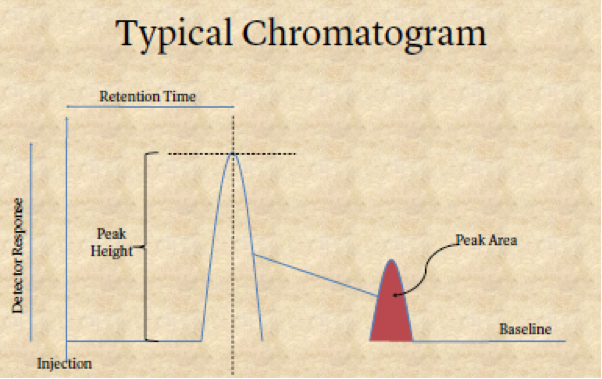
Separation must be proved. If it is not proved, then there can be no valid result. Proof of sufficient separation can only come from two sources: (1) knowing the column and the machine’s conditions, and (2) a chromatogram, which is a written record that proves separation has properly occurred, shows separation.
As a basic transitive property, without proof of sufficient separation, there can be no valid test and if the records of the analysis reveal there was no proof of separation, then this test cannot be valid.
There is no valid BAC.
One of the most basic fatal forensic flaws that I see all across America time and again is that the essential ethanol free blanks showed positive for ethanol. This is the second most frequent fatal forensic flaw that I see. In GC work, an ethanol free blank is a chromatogram that is used to prove that there is no carryover (contamination) of ethanol from one person’s test to another person’s test. If there is ethanol in the ethanol free blank that means that ethanol has carried over from someone else’s test. This means that the amount (the number) with the BAC is not correct. Having a blank ethanol free blank is an essential part of quality control. Blanks must be blank, meaning that the ethanol free blank must not contain any detected ethanol. If it does, then there is no valid result. Despite these clear statements in instructions (standard operating procedures) that ethanol free blanks shall be balnk, frequently analysts and Quality Assurance officers ignore the rules. Some will knowingly ignore the objective instrument data and write “Neg” meaning there was none at all detected. As can happen, the analyst can turn a blind eye to this fatal forensic flaw because to acknowledge that it is a problem would ruin a whole days work. This handwritten “Neg” can be proven to be not true. There is frequently ethanol detected as seen in the chromatogram below where there must be no ethanol. This fatal forensic flaw should have invalidated the BAC results for the batch. But instead of following the rules, they reported out the results in this batch as if all of the rules were met.
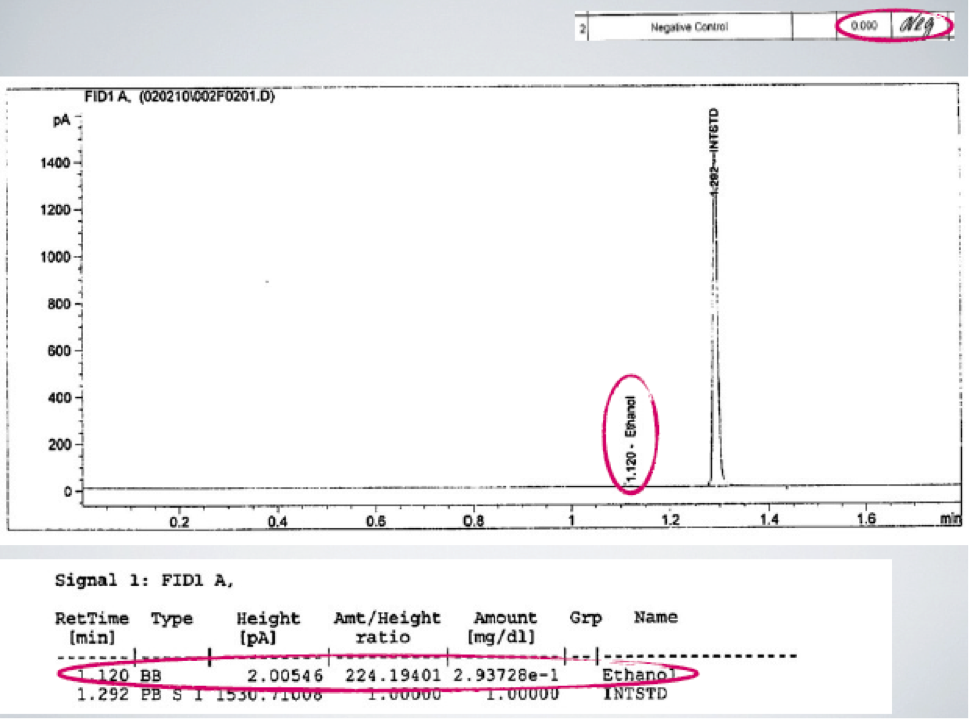
In the above case, the blank clearly shows that ethanol was detected in the ethanol free blank. Therefore, the BAC number attributed to any motorist cannot be said to come exclusively from that motorist alone. The BAC cannot be valid.
In conclusion, just a little bit of knowledge can go a long way in making sure that only acceptable forensic science makes its way into the courtroom.
For further information and training in GC techniques for forensic purposes, please go to www.ForensicChromatography.com



Brent Delapaz says:
Great article, if you have questions about how this applies to your situation give me a call. I’m an experienced San Antonio DWI Attorney