Simply put, the column is where the chromatographic magic occurs. It is how the complex mixture of the jumbled mess of “stuff” (for example, blood) is uniquely separated into its unique parts (hopefully if everything is done correctly). The variables of the column in conjunction with the conditions of the method are that which causes the separation of the complex mixtures. It is what causes the analyte of interest (the target compound you are looking for) to become separate from everything else.
Stated differently, it is how the analyst arrives at the qualitative measure of the analytical process. For example, how you know it is meth and nothing else.
It is primarily separation based upon boiling point and polarity. You are not expected to understand how that occurs at this point, but you need to be exposed to it.
Physically it is a coiled tube. What I am going to describe is that which is most frequently used in Gas Chromatography (GC) that defense attorneys, judges and prosecutors will encounter in our state crime labs. It is called a capillary column. Technically it is called a Wall Coated Open Tubular (WCOT) column. Within the inside of the tube is a thin layer coating. This inner coating is comprised of different chemicals in different thickness and made up of different compounds. This inner coating is called the stationary phase.
Capillary columns are a thin fused-silica (purified silicate glass) on the outside. They are typically 10-100 m in length and 250 µm inner diameter.
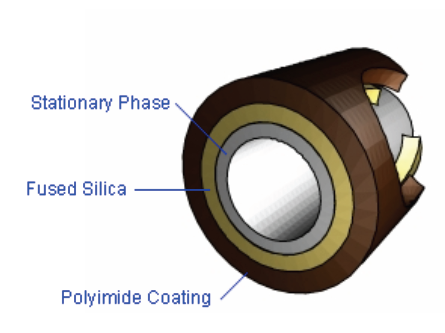

What the inner coating (stationary phase) is and its thickness as well as the length of the column will determine the ability of that column to be selective or specific as well as its sensitivity (definitions of those terms follow below).
Inner coatings (stationary phase) are most frequently made of polysiloxanes, which contain various substituent groups to change the polarity of the stationary phase. The nonpolar end of the spectrum is polydimethyl siloxane, which can be made more polar by increasing the percentage of phenyl groups on the polymer. For very polar analytes, polyethylene glycol (a.k.a. carbowax) is commonly used as the stationary phase. After the polymer coats the column wall or packing material, it is often cross-linked to increase the thermal stability of the stationary phase and prevent it from gradually bleeding out of the column.
Definitions:
Selective: Selectivity refers to the extent to which the method can be used to determine particular analytes in mixtures or matrices without interferences from other components of similar behavior. Selectivity gives an indication of how strongly the result is affected by other components in the sample. A selective test may be not a specific test due to cross-reactivity, interference, or codetermination.
- Sensitive: the quantitative measure is within the demonstrated linear dynamic range in that the unknown amount exceeds both the Limit of Detection and the Limit of Quantification but does not exceed the Limit of Linearity;
Specific: A test that reacts only with the analyte of interest and nothing else so that when there is a measure it is unique to that which is examined. It is not simply selective, which is an exhibition of a degree of preference for the substance tested for, but not necessarily unique.



